UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event reported):
(Exact Name of Registrant as Specified in Charter)
|
|
|
|
|
|
|
(State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
(Address of Principal Executive Offices, and Zip Code)
Registrant’s Telephone Number, Including Area Code
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
|
Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
|
Pre-commencement communication pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
|
Pre-commencement communication pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01. Other Events.
On September 15, 2020, EyePoint Pharmaceuticals, Inc. posted an updated corporate presentation on its website at www.eyepointpharma.com. A copy of the presentation is filed herewith as Exhibit 99.1 and is incorporated by reference herein.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
EyePoint Pharmaceuticals, Inc. |
||
|
|
|
|||
|
Date: September 15, 2020 |
|
By: |
|
/s/ Nancy Lurker |
|
|
|
Name: |
|
Nancy Lurker |
|
|
|
Title |
|
President and Chief Executive Officer |

CANTOR VIRTUAL HEALTHCARE CONFERENCE SEPTEMBER 15, 2020 NASDAQ: EYPT Exhibit 99.1

FORWARD LOOKING Various statements made in this presentation are forward-looking, and are inherently subject to risks, uncertainties and potentially inaccurate assumptions. All statements that address activities, events or developments that we intend, expect, plan or believe may occur in the future, including but not limited to statements about our expectations regarding the potential benefits of our partnerships and strategic alliances with other companies, as well as the timing and clinical development of our product candidates, including EYP-1901; and the potential for EYP-1901 as a vital, novel six-month treatment for serious eye diseases, including wet age-related macular degeneration, diabetic retinopathy and retinal vein occlusion; and our longer term financial and business goals, are forward-looking statements. Some of the factors that could cause actual results to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements are risks and uncertainties inherent in our business including, without limitation: the extent to which COVID-19 impacts our business; the effectiveness and timeliness of clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; our ability to achieve profitable operations and access to needed capital; fluctuations in our operating results; our ability to successfully produce sufficient commercial quantities of YUTIQ and DEXYCU and to successfully commercialize YUTIQ and DEXYCU in the U.S.; our ability to sustain and enhance an effective commercial infrastructure and enter into and maintain commercial agreements for YUTIQ and DEXYCU; the development of our YUTIQ line extension shorter-duration treatment for non-infectious uveitis affecting the posterior segment of the eye; potential off-label sales of ILUVIEN for non-infectious uveitis affecting the posterior segment of the eye; consequences of fluocinolone acetonide side effects for YUTIQ; consequences of dexamethasone side effects for DEXYCU; successful commercialization of, and receipt of revenues from, ILUVIEN for diabetic macular edema, or DME; Alimera’s ability to obtain additional marketing approvals and the effect of pricing and reimbursement decisions on sales of ILUVIEN for DME; Alimera’s ability to commercialize ILUVIEN for non-infectious uveitis affecting the posterior segment of the eye in the territories in which Alimera is licensed to do so; our ability to market and sell products; the success of current and future license agreements, including our agreement with Equinox Science; termination or breach of current license agreements, including our agreement with Equinox Science; our dependence on contract research organizations, contract sales organizations, vendors and investigators; effects of competition and other developments affecting sales of products; market acceptance of products; effects of guidelines, recommendations and studies; protection of intellectual property and avoiding intellectual property infringement; retention of key personnel; product liability; industry consolidation; compliance with environmental laws; manufacturing risks; risks and costs of international business operations; volatility of our stock price; possible dilution; absence of dividends; and other factors described in our filings with the Securities and Exchange Commission. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. We do not undertake any obligation to publicly update or revise our forward-looking statements even if experience or future changes makes it clear that any projected results expressed or implied in such statements will not be realized.

COMPANY OVERVIEW Ocular Disease Focus Portfolio of commercial- and clinical-stage assets targeting attractive areas of unmet need in ocular diseases Compelling Pipeline Includes EYP-1901 a potential six-month sustained release anti-VEGF treatment for wet age-related macular degeneration positioned for IND filing in Q4 2020 Commercial Revenue Customer demand for YUTIQ® and DEXYCU® franchises trending positive as the U.S. emerges from COVID-19 shut-downs Validated Technology Durasert® sustained-release technology has broad application across both internal programs and external partnerships ~$33M Cash at August 31, 2020

1 Alimera Sciences, Inc. owns worldwide rights to ILUVIEN® for DME and rights for YUTIQ® for non-infectious posterior uveitis in the EMEA with a royalty payable to EyePoint. 2 Rights for China, Hong Kong, Taiwan, Macau , Korea and certain SE Asia countries licensed to Ocumension with a royalty on sales payable to EyePoint 3 Excludes China, Hong Kong, Taiwan and Macau OCULAR DISEASE FOCUSED PIPELINE Program Preclin. Phase 1 Phase 2 Phase 3 Commercial Rights DEXYCU® post-operative inflammation following ocular surgery WW2 YUTIQ® - three-year treatment for chronic non-infectious uveitis affecting the posterior segment U.S.1,2 YUTIQ® 50 short duration treatment for chronic non-infectious uveitis affecting the posterior segment WW EYP-1901 – six-month anti-VEGF treatment for wet AMD WW3 Durasert® Technology Durasert® Partners Preclin. Phase 1 Phase 2 Phase 3 Commercial ILUVIEN/Alimera Sciences – DME Undisclosed – Ophthalmology Undisclosed - Non-ophthalmology Undisclosed - Other small molecule

Four FDA-Approved Products with Multiple Programs in Development DURASERT® - Proven Sustained Release Delivery Sustained-release delivery of small molecule drugs to the back of the eye Release profile allows design of treatment duration from months to years Administration during Physician office visit 1 Durasert® non-erodible technology 2Durasert® bioerodible technology Approved products1/Indications: YUTIQ® (2018, EyePoint) - Posterior Segment Uveitis ILUVIEN® (2014, Alimera) - DME RETISERT ® (2005, B&L) - Uveitis VITRASERT® (1996, B&L) - CMV retinitis Development Candidates: EYP-19012 (EyePoint) – Wet AMD YUTIQ® 501 (EyePoint) - Posterior Segment Uveitis Partner programs

EYP 1901 - Six-Month Sustained-Release Anti-VEGF Product Candidate Opportunity in Wet AMD, Diabetic Retinopathy, and Retinal Vein Occlusion

EYP-1901 Product Candidate Overview Anti-VEGF intravitreal therapy with sustained, consistent delivery of drug over at least 6 months. Initial clinical target – wet AMD Utilizes Durasert technology and an anti-VEGF small molecule, vorolanib – a tyrosine kinase inhibitor (TKI) Vorolanib previously studied as an oral agent for wet AMD through Phase 2, Strong efficacy signal and no significant ocular adverse events Efficacy and preliminary safety study completed in a laser CNV mini pig model with low doses of EYP-1901 Results: dose-related efficacy and no clinically observed toxicity Non-GLP rabbit PK and safety study of EYP-1901 demonstrate drug levels in vitreous and retina/choroid significantly above the IC50 for VEGFR GLP toxicology program underway with 6-month data expected in October 2020 IND filing on track for Q4 2020

EYP-1901 Vorolanib– Mechanism of Action at Receptor Vorolanib

Oncologist. 2019 Apr; 24(4): 455–e121. Phase I, First‐in‐Human, Dose‐Escalation Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Vorolanib in Patients with Advanced Solid Tumors EYP-1901 Vorolanib Background Vorolanib - developed on the same chemical scaffold as sunitinib Targets all 3 isoforms of VEGFR and PDGFR Designed to improve the safety profile while maintaining the efficacy of sunitinib X-82 - oral dosage form of vorolanib - Phase 1 and 2 wet AMD studies completed by Tyrogenex EYP-1901 - intravitreal formulation of vorolanib with Durasert

IC50 Data Compared to Sunitinib The inhibition constant of sunitinib for VEGFR (Ki) is reported to be low (5 ng/g), an indication on strong inhibition. Since Ki is related to IC50, similar inhibition (Ki) is expected for vorolanib EYP-1901 Vorolanib Background The most important targets of ocular neovascularization are strongly inhibited by vorolanib and sunitinib with comparable IC50 values Biochemical Selectivity (IC50 in µM) ID VEGFR PDGFR Sunitinib 0.043 0.16 Vorolanib 0.052 0.26 Biochemical Selectivity (IC50, ng/g) Sunitinib 22.9 85.1 Vorolanib 22.9 114.3

Phase 1 Trial – open label, 24 weeks, dose escalation, no control, oral delivery. 80 % of eyes enrolled previously treated. 4 eyes treatment naïve. Study completed by Tyrogenix, Inc. 1Jackson TL et al. JAMA Ophthalmology July 2017 Volume 135, Number 7, 2017 EYP-1901 Vorolanib (X-82) Clinical Study – Ph1 Despite low retreatment rates, BCVA was maintained to within 4 letters of baseline at the 24-week endpoint, or improved in all but 1 participant 60% of patients (15 of 25) required no rescue injections while on 24-week study Mean OCT thickness in completers was reduced by -50 +/- 97 µm Mean change was +3.8 +/- 9.6 letters (n=25 completers) Mean time to the first rescue injection was 130 days in the 10 participants who completed the study and required an injection Mean OCT thickness in treatment-naïve patients was reduced by ~80 µm Visual Acuity (BCVA) Anti—VEGF Rescue Injections Central Retinal Thickness

Phase 1 Trial - Rescue Injections EYP-1901 Vorolanib (X-82) Clinical Study – Ph1 25 of 35 completed at the 6 Mo Follow up
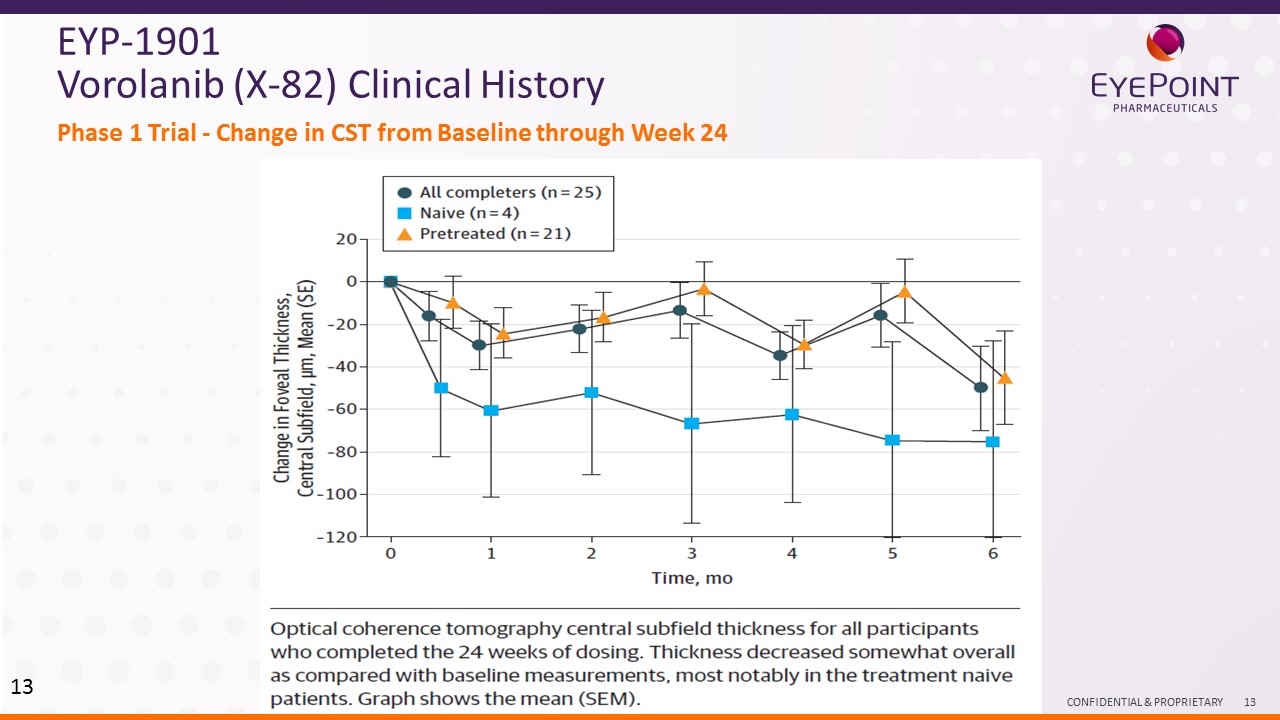
Phase 1 Trial - Change in CST from Baseline through Week 24 EYP-1901 Vorolanib (X-82) Clinical History

*Normalized for number of months on study. Study completed by Tyrogenex, Inc. Phase 2b Trial (Apex) in wAMD – Oral Administration - Number of Anti-VEGF Injections EYP-1901 Vorolanib (X-82) Clinical Study Ph2 For subjects followed ≥ 6 months, number of anti-VEGF injections per year* Placebo n=33 50 mg n=34 100 mg n=30 200 mg n=26 Median 9.0 6.1 5.8 4.6 Number of Patients w/ no rescue 2.6% 7.5% 10.3% 20.5% Pre-defined rescue criteria with intravitreal anti-VEGF therapy Any increase in fluid on OCT compared to Screening Visit 2 (~14 days after an IVT injection) New or increased macular hemorrhage by fundus photography Double masked study – investigators unaware of treatment v control Less rescue vs placebo for all doses. Numerically smallest for 200 mg dose (~118 ng/g SS). No ocular tox.

EYP-1901 Phase 1 Study Plan Approximately 20 patients with wet AMD responsive to previous anti-VEGF therapy enrolled in US sites Primary endpoint - safety (AE rates and severity); BCVA and central subfield thickness secondary Rescue with anti-VEGF’s if necessary according to industry standard clinical criteria EYP-1901 dosed 1-2 weeks following the last anti-VEGF injection Three dose levels. Follow up though 12 months (6-month timepoint is key readout) Planned expansion with additional patients to provide additional efficacy and safety data Open label, dose-escalation, no control (results to be monitored on an ongoing basis)

EYP-1901 - Next Steps and Development Plan Q3 2020 Q4 2020 1H 2021 GLP Toxicology Study Phase 1 Trial Type B Pre-IND meeting with FDA in January 2020 GLP toxicology study initiated in March 2020–unaffected by COVID-19 shut-downs IND filing in Q4 2020 with Phase 1 initiation to follow Initial data expected in 2H of 2021 IND Filing

Commercial Programs

Postoperative inflammation following ocular surgery Chronic non-infectious uveitis affecting the posterior segment of the eye Single long-lasting treatment compared with complicated eyedrop regimen Permanent and specific J-Code with solid reimbursement experience Co-Promotion with ImprimisRX in place for U.S. market Addresses limitations of short-acting standard of cares to decrease uveitis flares Permanent and specific J-Code

YUTIQ® - 3 YEAR TREATMENT FOR CHRONIC NONINFECTIOUS UVEITIS Patients in the U.S. with Chronic Non-infectious Posterior Segment Uveitis ~60K–100K ~30,000 new cases of blindness per year in the U.S. 3rd leading cause of blindness in the U.S. Noninfectious uveitis is inflammation of the uveal tract and adjacent structures Spontaneous and uncontrolled uveitic flares can lead to severe vision loss or blindness Disease is often lifelong and YUTIQ provides an effective three-year treatment option Market Potential Patient Experience

YUTIQ Customer Demand Quarterly Trend Units Shipped Q3’20 data through August 2020 Month 1 and 2 of Quarter Month 3 of Quarter Covid-19 Closures Q3 Demand Through August 31

U.S. Cataract Surgery Large and Growing * Based upon company estimates for 2018. Source: imaged from the American Optometric Association. DEXYCU® CATARACT SURGERY MARKET Cataract Surgeries in 2018 3.8 Million* 8% annual growth rate in the U.S. Most performed surgery in the U.S. Baby boomers; longer life expectancy with greater access to healthcare Improvements in technology Improved outcomes Physician Perspective Poor patient compliance with drop regimen can lead to poor outcomes Patient call backs are time consuming and disruptive to physician office Patients/caregivers are frustrated and confused with regimen

DEXYCU Customer Demand Quarterly Trend Units Shipped Q3’20 data through end of August First 2 months of Quarter Third month of Quarter Covid-19 Closures ~3K units is largest 2-month customer demand since launch

DEXYCU - EXPANDING PRODUCT REACH ImprimisRX - Commercial Alliance, August 2020 Focus on volume-based agreements with ambulatory surgical centers and integrated healthcare networks Latest strategic purchase and marketing agreement secured with Vantage Outsourcing in August 2020 One of Largest Integrated Delivery Systems in the U.S.

COMPANY OVERVIEW Ocular Disease Focus Portfolio of commercial- and clinical-stage assets targeting attractive areas of unmet need in ocular diseases Compelling Pipeline Includes EYP-1901 a potential six-month sustained release anti-VEGF treatment for wet age-related macular degeneration positioned for IND filing in Q4 2020 Commercial Revenue Customer demand for YUTIQ® and DEXYCU® franchises trending positive as the U.S. emerges from COVID-19 shut-downs Validated Technology Durasert® sustained-release technology has broad application across both internal programs and external partnerships